Abstract
Background: Delayed or failed engraftment after adult UCBT is a major contributor to the morbidity, mortality and resource utilization associated with this procedure. Increasing the number of hematopoietic stem and progenitor cells within the cord blood graft may overcome this limitation. NiCord, developed and manufactured by Gamida Cell, is an ex vivo-expanded, cryopreserved graft derived from an entire cord blood unit (CBU). In a pilot study, NiCord transplanted with a second, unmanipulated CBU was shown to shorten time to neutrophil engraftment and provide long-term hematopoiesis (Horwitz M, JCI 2014). In another study, NiCord UCBT demonstrated clinical benefit by reducing the incidence of severe infections as well as the duration of hospitalization during the first 100 days following transplantation (Anand S BBMT 2017). With confirmation that NiCord expands both hematopoietic stem and progenitor cells, we conducted a multicenter study using NiCord as a single stem cell graft.
Methods: The study objective was to assess safety and efficacy of NiCord as a single, ex vivo expanded stem cell graft. The primary endpoint was to assess the cumulative incidence of NiCord-derived neutrophil engraftment. Thirty-five evaluable patients, median age 44 (13-63) with ALL (n=9), AML (n=16), MDS (n=7), CML (n=2) or Hodgkin lymphoma (n=1) were transplanted at 10 sites in the USA, EU, and Singapore. 79% had intermediate-high risk disease by Disease Risk Index. All patients received myeloablative conditioning with a TBI-based (n=14) or chemotherapy-only (n=21) regimen. Graft vs. host disease (GvHD) prophylaxis consisted of MMF for a minimum of 60 days and tacrolimus or cyclosporine for a minimum of 180 days. HLA matching was 4/6 (n=25), 5/6 (n=8) or 6/6 (n=2).
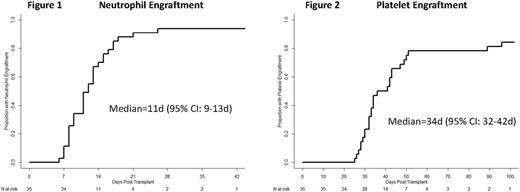
Results: NiCord processing resulted in a median 32-fold (20-52 fold) increase in CD34+ cells relative to that reported by the cord blood bank prior to cryopreservation. This translated to a median CD34+ cell dose of 6.3 x 106/kg (1.4-14.9 x 106/kg). The cumulative incidence of engraftment at day 42 was 94%. Two patients experienced secondary graft failure; one at day 40 due to HHV6 infection, and another at day 260 due to an overwhelming, lethal adenovirus infection. Neutrophil engraftment occurred at a median of 11 days (95% CI: 9-13 days)(Figure 1). Platelet engraftment occurred at a median 34 days (95% CI:32-42 days)(Figure 2). Full donor whole blood chimerism (≥ 95%) was observed in 100% of engrafted patients by day 100 following transplantation. The incidence of grade II-IV and grade III/IV acute GvHD was 48.6% and 12.2%, respectively. Cumulative incidence of all chronic GvHD was 45%, and moderate/severe cGvHD 11.5% at 1 year following transplantation. Median CD3+, CD4+, and CD19+ lymphocyte counts were 286/µl, 183/µl, and 147/µl at day 100, and 479/µl, 290/µl, and 446/µl at 6 months, respectively. The incidence of grade 2-3 bacterial (n=9) or grade 3 fungal (n=0) infections was 23.9% by day 100. With a median follow-up of 13 months (range 1-35 months), the 6-month and 2-year non-relapse mortality was 12.8% and 20.3%, respectively. The cumulative incidence of relapse was 27% at 2 years. The Kaplan-Meier estimate of overall survival at 6 months and 2 years was 80.5% and 50.8%, respectively.
Conclusions: The rapid engraftment kinetic coupled with a low graft failure rate and durable hematopoiesis suggests a favorable safety profile of NiCord as a single umbilical cord blood graft. Ex vivo expansion of the CBU resulted in a CD34+ cell dose comparable to adult mobilized peripheral blood stem cell grafts. Compared to a recently reported multi-center phase II study of adult, unmanipulated myeloablative double UCBT (Barker J, Br. J. Haem 2014), NiCord markedly reduced the median time to neutrophil recovery (11 days vs. 22 days) and platelet recovery (34 days vs. 49 days). Transplantation of NiCord appears not to adversely affect cellular immune recovery. Quantitative assessment of neutrophil, T-cell, and B-cell recovery is comparable to that observed following adult matched unrelated donor transplantation. We conclude that UCBT using NiCord has the potential to broaden accessibility to stem cell transplantation and to become a graft of choice for patients without a matched donor. A global randomized phase III study of NiCord vs. standard UCBT is ongoing (NCT02730299).
Jagasia: Therakos: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Mallinckrodt: Consultancy. Wagner: Magenta Therapeutics: Research Funding; Novartis: Research Funding. Kuball: Gadeta (www.gadeta.nl): Consultancy, Equity Ownership, Patents & Royalties: on gdT cells and receptors and isolation strategies , Research Funding; Miltenyi: Research Funding. Majhail: Anthem, Inc.: Consultancy; Sanofi: Honoraria. Montesinos: Celgene Corporation: Honoraria, Research Funding. Kurtzberg: Gamida Cell: Research Funding. Sanz: Gamida Cell: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.